HRT active ingredients
HRT active ingredients are the hormones used in contraceptives and hormone therapies. This page is dedicated to listing abstracts from medical research papers relating to the active ingredients used in hormonal products: Hormone Replacement Therapy (HRT); Menopausal Hormone Therapy (MHT); Hormone Therapy (HT), Contraceptives, Bioidentical Hormone Replacement Therapy (BHRT).
It is meant to serve as a reference source for you to do your own research into the effects and side effects of these active ingredients. Please follow the links to the source for further reading.
When choosing a hormonal supplement, you must decide if you want to use a supplement that contains hormones that are bio-identical, meaning their molecular structure is identical to those which the body makes, or if you want to go the synthetic route and use a hormone that has been manufactured and had its molecular structure altered. We do not advocate using the latter.
"Many synthetic drugs are made patentable simply by changing a few atoms of the natural substance. This may sound harmless enough, but the addition or subtraction of a few atoms of a molecule can make a big difference in their effects on the body. This holds especially true with hormones. Tiny amounts can create major effects on the body. For example, the molecular difference between testosterone and estradiol (a form of estrogen) is one hydrogen atom and a couple of double bonds. Amazing! Adding or subtracting one hydrogen atom at a specific place on a molecule can make the difference between a man and a woman!"
 |
 |
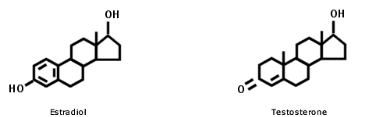
Conjugated Equine Estrogens [CEE]
Wyeth Ayerst produced the first conjugated oestrogen called Premarin, standing for 'Pregnant Mare's Urine'. It is, as the name suggests, made from pregnant mare's urine. The pregnant horses, some say as many as 80,000, are kept tethered in stalls for six months of the year, fed little water to concentrate the urine, which is collected in bags held under the horses tails. The urine is then processed to yield various hormones, only 40% being human hormones, the potent remainder being horses hormones.
Common synthetic conjugated estrogens used in HRT:
- sodium estrone sulfate
- sodium equilin sulfate,
- sodium 17α- dihydroequilin sulfate
- sodium 17α-estradiol sulfate
- sodium 17β-dihydroequilin sulfate
- sodium 17α- dihydroequilenin sulfate
- sodium 17β-dihydroequilenin sulfate
- sodium equilenin sulfate
- sodium 17β-estradiol sulfate
- sodium ∆8,9 -dehydroestrone sulfate.
The Women's Health Initiative Memory Study: findings and implications for treatment
“There were two study arms, one involving 4532 postmenopausal women who received continuous combined oestrogen (conjugated equine oestrogens [CEE] plus medroxyprogesterone acetate) or placebo, and the other involving 2947 hysterectomised women randomised to continuous unopposed CEE or placebo. All participants were age 65 years or older. CEE with or without medroxyprogesterone acetate, given to women age 65 years and older, does not protect against dementia or cognitive decline, but substantially increases the risk of dementia of any cause and cognitive decline.”
-The Women's Health Initiative Memory Study: findings and implications for treatment Craig, Michael C et al. The Lancet Neurology, Volume 4, Issue 3, 190 - 194-
Cell Mol Life Sci. 2005 Feb
“..findings from the Women s Health Initiative (WHI) have raised considerable concern over prolonged use of opposed and unopposed oral conjugated equine estrogen (CEE), given the increased risk of serious adverse effects, including stroke and venous thromboembolic complications. Furthermore, results from the WHI Memory Study (WHIMS) indicated that over 5 years of therapy with Prempro impaired performance on global cognitive tests and nearly doubled the risk of dementia.”
- Gleason CE, Cholerton B, Carlsson CM, Johnson SC, Asthana S. Neuroprotective effects of female sex steroids in humans: current controversies and future directions. Cell Mol Life Sci. 2005 Feb;62(3):299-312. doi: 10.1007/s00018-004-4385-z. PMID: 15723166.-
11-Year WHI Data Show Increase in More Advanced Breast Cancers
Medscape, Oct 19, 2010.
“With median of 11 years of follow-up now in hand, investigators from the landmark Women Health Initiative (WHI) confirm that estrogen-plus-progestin hormone therapy is associated with greater breast cancer incidence than placebo. In short, they now say that the effect is long-term.
Findings
In the WHI, 16,608 postmenopausal American women, 50 to 79 years of age, who had not undergone hysterectomy were randomly assigned to receive either combined conjugated equine estrogens 0.625 mg/day plus medroxyprogesterone acetate 2.5 mg/day, or placebo.
After the original trial completion date, reconsent was required for continued follow-up for breast cancer incidence, and was obtained from 12,788 (83%) of the surviving participants, report the authors, led by Rowan T. Chlebowski, MD, PhD, from the Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center in Torrance, California.
The WHI authors performed intention-to-treat analyses and found that combined estrogen-plus-progestin hormone therapy increased the incidence of invasive breast cancer, compared with placebo (385 cases [0.42% per year] vs 293 cases [0.34% per year]).”
-11-Year WHI Data Show Increase in More Advanced Breast Cancers - Medscape - Oct 19, 2010.
Nick Mulcahy-
RX List: Cenestin: synthetic conjugated estrogens
FDA Data Sheet: ENJUVIA™: synthetic conjugated estrogens
Desogestrel
Desogestrel is a synthetic progestin used in contraception.
Pulmonary embolism in a healthy woman using the oral contraceptives containing desogestrel.
Obstetrics & Gynecology Science; 2017 Mar
"Venous thromboembolism is well known as one of the rare but serious adverse effects of combined oral contraceptives (COCs). The COCs with third and fourth generation progestogens were found to have higher risk of venous thrombosis than those with second generation progestogens. We present a case of pulmonary embolism in a 23-year-old nulligravid woman who was using COCs containing the third generation progestogen (desogestrel)."
-Park MJ, Jeon GH: Pulmonary embolism in a healthy woman using the oral contraceptives containing desogestrel. Obstet Gynecol Sci. 2017 Mar;60(2):232-235. doi: 10.5468/ogs.2017.60.2.232. Epub 2017 Mar 16.-
Molecules, 2021 Feb 18
“New generations of OC pills are characterized by lower estrogen content and by newer progestins, like desogestrel, gestodene, cyproterone, and drospirenone with lower androgenicity than past generation pills. They have been introduced to reduce severe adverse effects of OC use, especially thromboembolism, and other cardiovascular diseases. However, these new OC preparations are still associated with the risk of pulmonary embolism, myocardial infarction, thrombotic stroke and VTE.”
“Pills had variable progestin components: gestodene (43.0%), drospirenone (24.0%), desogestrel (12.0%), levonorgestrel (10.0%), cyproterone (4.0%), dienogest (3.0%), clormadinone (2.0%), and nomegestrolo acetate (2.0%). Overall, among the 100 OC-users second generation pill preparations (containing levonorgestrel) were used by 10.0%, third generation pill preparations (having gestodene or desogestrel as progestin) by 55.0%; and fourth generations pills (containing drospirenone, cyproterone, dienogest, clormadinone and nomegestrolo acetate) by 35.0% of women. Preparations containing progestogens with the highest risk of VTE according to recent evidence i.e., those including desogestrel, cyproterone, and drospirenone were used by 40.0% of OC-users.”
“The risk of VTE associated to OC use is of particular concern and has been recently investigated in a total of 10,562 cases of thromboembolism. In respect to no exposure to OCs in the previous year, exposure to OC containing desogestrel had increased risk with OR of 4.28, cyproterone 4.27, drospirenone 4.12, gestodene 3.64, norethisterone 2.56, norgestimate 2.53, and levonorgestrel 2.38. Similarly, another study found that the relative risk of VTE for combined oral contraceptives with 30–35 μg ethinyl-estradiol and gestodene, desogestrel, cyproterone acetate, or drospirenone were similar and about 50%–80% higher than for combined oral contraceptives with levonorgestrel.”
-Cauci S, Xodo S, Buligan C, Colaninno C, Barbina M, Barbina G, Francescato MP. Oxidative Stress Is Increased in Combined Oral Contraceptives Users and Is Positively Associated with High-Sensitivity C-Reactive Protein. Molecules. 2021 Feb 18;26(4):1070. doi: 10.3390/molecules26041070. PMID: 33670593; PMCID: PMC7921945.-
Oxidative Stress in Female Athletes Using Combined Oral Contraceptives
“The markedly elevated oxidative stress we revealed in OC-user athletes could be detrimental to physical activity and elevate cardiovascular risk (as thromboembolism). Further research is needed to extend our results, to clarify the biochemical pathways leading to increased hydroperoxides (mainly lipid peroxides) and reduced antioxidant defense, and to elucidate the potential effects on athletic performance. OC use should be considered when developing gender-focused strategies against oxidative stress.”
-Cauci S, Buligan C, Marangone M, Francescato MP. Oxidative Stress in Female Athletes Using Combined Oral Contraceptives. Sports Med Open. 2016 Dec;2(1):40. doi: 10.1186/s40798-016-0064-x. Epub 2016 Sep 21. PMID: 27747795; PMCID: PMC5031583.-
Diethylstilbestrol
Diethylstilbestrol (DES) Exposure and Cancer
National Cancer Institute
"Diethylstilbestrol (DES) is a synthetic form of the female hormone estrogen. It was prescribed to pregnant women between 1940 and 1971 to prevent miscarriage, premature labor, and related complications of pregnancy. The use of DES declined after studies in the 1950s showed that it was not effective in preventing these problems, although it continued to be used to stop lactation, for emergency contraception, and to treat menopausal symptoms in women.
In 1971, researchers linked prenatal (while in the womb, or in utero) DES exposure to a type of cancer of the cervix and vagina called clear cell adenocarcinoma in a small group of women. Soon after, the Food and Drug Administration (FDA) notified health care providers throughout the country that DES should not be prescribed to pregnant women. The drug continued to be prescribed to pregnant women in Europe until 1978.
DES is now known to be an endocrine-disrupting chemical, one of a number of substances that interfere with the endocrine system to potentially cause cancer, birth defects, and other developmental abnormalities. “
-Diethylstilbestrol (DES) Exposure and Cancer was originally published by the National Cancer Institute, Updated January 31, 2025-
International Agency for Research on Cancer; 2012
“Diethylstilbestrol is a synthetic non-steroidal estrogen that was historically widely used to prevent potential miscarriages by stimulating the synthesis of estrogen and progesterone in the placenta (in the United States of America, especially from the 1940s to the 1970s) (Rogers & Kavlock, 2008). It was also used for the treatment of symptoms arising during the menopause and following ovariectomy, and for senile (atrophic) vaginitis and vulvar dystrophy. Diethylstilbestrol was used as a postcoital emergency contraceptive (‘morning-after pill’). It has also been used for the prevention of postpartum breast engorgement, for dysfunctional menstrual cycles, and for the treatment of female hypogonadism.
Diethylstilbestrol is now rarely used to treat prostate cancer because of its side-effects. It is occasionally used in postmenopausal women with breast cancer.
Diethylstilbestrol was also used as a livestock growth stimulant.”
-IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Pharmaceuticals. Lyon (FR): International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100A.) DIETHYLSTILBESTROL. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK304340/-
Drospirenone
National Center for Biotechnology Information (2024).
“Drospirenone is a steroid lactone and a 3-oxo-Delta(4) steroid. It has a role as a contraceptive drug, an aldosterone antagonist and a progestin.
Drospirenone is a synthetic progestin commonly found in the popular oral contraceptive, Yaz in combination with [Ethinyl estradiol]. Most recently, it was approved by both Health Canada and the FDA in combination with [Estetrol] as an oral contraceptive therapy. Aside from its contraceptive effects, drospirenone is used with estrogens to control acne and premenstrual dysphoric disorder (PMDD). Drospirenone has been the subject of widespread safety concern due to the possibility of an increased risk of venous thromboembolism associated with its use. In 2012, however, a safety statement by the FDA concluded that the increase in the risk of thromboembolism resulting from the use of drospirenone remains unclear, as studies regarding this risk are conflicting. Some studies have demonstrated a significantly increased risk and some demonstrating no risk of thromboembolic events. In its statement, the FDA has mentioned that increased risk of venous thromboembolism with oral contraceptives such as drospirenone exists but remains lower than the risk of this condition during pregnancy and during the postpartum period, and this should be considered when assessing potential risks of hormonal contraceptive use.”
-National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 68873, Drospirenone. Retrieved December 16, 2024 from https://pubchem.ncbi.nlm.nih.gov/compound/Drospirenone.-
FDA Drug Safety Communication; 2012
“The U.S. Food and Drug Administration (FDA) has completed its review of recent observational (epidemiologic) studies regarding the risk of blood clots in women taking drospirenone-containing birth control pills. Drospirenone is a synthetic version of the female hormone, progesterone, also referred to as a progestin. Based on this review, FDA has concluded that drospirenone-containing birth control pills may be associated with a higher risk for blood clots than other progestin-containing pills.”
BMJ. 2009 Aug 13
“Currently available oral contraceptives increased the risk of venous thrombosis fivefold compared with non-use (odds ratio 5.0, 95% CI 4.2 to 5.8). The risk clearly differed by type of progestogen and dose of oestrogen. The use of oral contraceptives containing levonorgestrel was associated with an almost fourfold increased risk of venous thrombosis (odds ratio 3.6, 2.9 to 4.6) relative to non-users, whereas the risk of venous thrombosis compared with non-use was increased 5.6-fold for gestodene (5.6, 3.7 to 8.4), 7.3-fold for desogestrel (7.3, 5.3 to 10.0), 6.8-fold for cyproterone acetate (6.8, 4.7 to 10.0), and 6.3-fold for drospirenone (6.3, 2.9 to 13.7). The risk of venous thrombosis was positively associated with oestrogen dose. We confirmed a high risk of venous thrombosis during the first months of oral contraceptive use irrespective of the type of oral contraceptives.
Conclusions: Currently available oral contraceptives still have a major impact on thrombosis occurrence and many women do not use the safest brands with regard to risk of venous thrombosis.”
-van Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. BMJ. 2009 Aug 13;339:b2921. doi: 10.1136/bmj.b2921. PMID: 19679614; PMCID: PMC2726929.-
Group seeks re-vote on birth control clot risk
Anna Yukhananov, January 13, 2012
"The FDA asked outside experts in December to discuss the safety of birth control that contains the compound drospirenone, including Bayer's Yaz and Yasmin.
The panel decided by a four-vote margin that the benefit of pregnancy prevention from these pills outweighed their risk of dangerous blood clots.
But according to court and public documents, three of the FDA's 26 advisers had research or financial ties to Bayer. A fourth adviser had a connection to a manufacturer of generic copies of Yaz, Barr Laboratories, now part of Teva Pharmaceuticals.
All four of these advisers voted that the drugs' benefits outweighed risks, meaning the pills could stay on the market, according to the Project on Government Oversight (POGO).
The FDA is not required to follow the recommendations of its advisers, but often does, leading to scrutiny of who is providing advice.
"The American public must be able to trust that the FDA and its advisory committees are making decisions based on science, not industry influence," POGO Executive Director Danielle Brian said.
The group sent a letter to FDA Commissioner Margaret Hamburg this week, asking her to discard the old vote and convene a new advisory committee on the pills' safety."
-Reuters; healthcare pharmaceuticals; Anna Yukhananov January 13, 2012-
Dydrogesterone
Anticancer Res. 2004 May-Jun
"It was concluded that the progestogen Duphaston and its 20-dihydro-metabolite are potent inhibitory agents on sulfatase and 17beta-HSD activities in breast cancer cells. Duphaston is a progestogen with properties similar to the endogenous progesterone. The data open interesting perspectives to study the biological responses of these progestogens in clinical trials of patients with breast cancer."
-Chetrite GS, Thole HH, Philippe JC, Pasqualini JR. Dydrogesterone (Duphaston) and its 20-dihydro-derivative as selective estrogen enzyme modulators in human breast cancer cell lines. Effect on sulfatase and on 17beta-hydroxysteroid dehydrogenase (17beta-HSD) activity. Anticancer Res. 2004 May-Jun;24(3a):1433-8. PMID: 15274306.-
Dydrogesterone after 60 years: a glance at the safety profile
Gynecol Endocrinol. 2022 Apr
"A total of 32 relevant clinical studies were identified. Results were reported in the context of overall adverse events (AEs) and segregated according to various progesterone-deficient conditions. AEs concerning breasts (breast cancer risk), the endometrium (endometrial cancer risk), venous thromboembolism risk, and cardiovascular risk were found to be minimal when dydrogesterone was used as part of a menopausal hormone therapy regimen lasting ≤260 weeks. Vagina-related AEs, such as bleeding, discharge, irritation, and difficult coitus, occurred less frequently with dydrogesterone when used as luteal phase support in the context of assisted reproductive techniques (ARTs). However, other common AEs, such as headache, dizziness, abdominal pain, flatulence, and nausea, occurred more frequently with dydrogesterone. No maternal complications or congenital anomalies could be linked to dydrogesterone usage during ARTs or during early pregnancy to prevent recurrent miscarriages. Studies on dydrogesterone in endometriosis and premenstrual syndrome remain scarce."
-Ott J, Egarter C, Aguilera A. Dydrogesterone after 60 years: a glance at the safety profile. Gynecol Endocrinol. 2022 Apr;38(4):279-287. doi: 10.1080/09513590.2021.2016692. Epub 2021 Dec 20. PMID: 34927507.-
J Matern Fetal Neonatal Med. 2024 Dec
"Oral dydrogesterone 20 mg/day could not prevent miscarriages in women with threatened miscarriage."
-Kuptarak A, Phupong V. Oral dydrogesterone for prevention of miscarriage in threatened miscarriage: a randomized, double-blind, placebo-controlled trial. J Matern Fetal Neonatal Med. 2024 Dec;37(1):2333929. doi: 10.1080/14767058.2024.2333929. Epub 2024 Apr 3. PMID: 38570191.-
Gestodene
"Gestodene (17 alpha-ethynyl-13 beta-ethyl-17 beta-hydroxy-4, 15-gonadien-3-one) is the most potent synthetic progestin currently available and it is widely used as a fertility regulating agent in a number of contraceptive formulations.."
"Gestodene was discovered in 1975 and was introduced for medical use, specifically in birth control pills, in 1987. It was subsequently introduced for use in menopausal hormone therapy as well. Gestodene is sometimes referred to as a "third-generation" progestin. It is marketed in birth control pills widely throughout the world, whereas it is available for use in menopausal hormone therapy only a few countries. Gestodene is not approved in the United States."
“New generations of OC pills are characterized by lower estrogen content and by newer progestins, like desogestrel, gestodene, cyproterone, and drospirenone with lower androgenicity than past generation pills [59]. They have been introduced to reduce severe adverse effects of OC use, especially thromboembolism, and other cardiovascular diseases [11]. However, these new OC preparations are still associated with the risk of pulmonary embolism, myocardial infarction, thrombotic stroke and VTE [2,59]."
“This study adds to the existing evidence that OC use alters the oxidative homeostasis and modifies low-grade inflammatory status of young women. We found very high oxidative stress in the vast majority of OC-users (77% of them had FORT ≥ 400 Units). The strong positive relationship, that we observed in healthy women, between oxidative stress and basal chronic inflammation has several potential implications. Elevation of both parameters can potentially concur in OC side effects like the increased risk of CVDs, cancer and other diseases, posing the question of medical eligibility criteria for contraceptive use [75,76], in particular for women affected by major diseases like high risk of CVDs, having had cancer or with major immune/inflammatory diseases like multiple sclerosis [77], diabetes [45,78] or HIV infection [79,80]. It remains to be determined whether oxidative stress or hsCRP elevation is the main driver of CVD risk and whether these two conditions are synergistic.”
-Cauci S, Xodo S, Buligan C, Colaninno C, Barbina M, Barbina G, Francescato MP. Oxidative Stress Is Increased in Combined Oral Contraceptives Users and Is Positively Associated with High-Sensitivity C-Reactive Protein. Molecules. 2021 Feb 18;26(4):1070. doi: 10.3390/molecules26041070. PMID: 33670593; PMCID: PMC7921945.-
“Currently available oral contraceptives increased the risk of venous thrombosis fivefold compared with non-use (odds ratio 5.0, 95% CI 4.2 to 5.8). The risk clearly differed by type of progestogen and dose of oestrogen. The use of oral contraceptives containing levonorgestrel was associated with an almost fourfold increased risk of venous thrombosis (odds ratio 3.6, 2.9 to 4.6) relative to non-users, whereas the risk of venous thrombosis compared with non-use was increased 5.6-fold for gestodene (5.6, 3.7 to 8.4), 7.3-fold for desogestrel (7.3, 5.3 to 10.0), 6.8-fold for cyproterone acetate (6.8, 4.7 to 10.0), and 6.3-fold for drospirenone (6.3, 2.9 to 13.7). The risk of venous thrombosis was positively associated with oestrogen dose. We confirmed a high risk of venous thrombosis during the first months of oral contraceptive use irrespective of the type of oral contraceptives.
Conclusions: Currently available oral contraceptives still have a major impact on thrombosis occurrence and many women do not use the safest brands with regard to risk of venous thrombosis.”
-van Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. BMJ. 2009 Aug 13;339:b2921. doi: 10.1136/bmj.b2921. PMID: 19679614; PMCID: PMC2726929.-
17α-hydroxyprogesterone caproate
17α-hydroxyprogesterone caproate (17-OHPC) is a synthetic progestogen
17OHP is a naturally occurring metabolite of progesterone it's fine. But often 17OHP caproate is used, which is synthetic.
“17α-hydroxyprogesterone caproate (17-OHPC) is a synthetic progestogen. The human body does not make the caproate molecule….
The abbreviation 17P has been used by many to refer to 17-OHPC. This has been unfortunate because the term 17P does not convey information about the presence of the caproate molecule. Indeed, 17P has also been used to refer to 17α-hydroxyprogesterone (17OHP), which is a naturally occurring steroid produced by the ovary with weak progestational activity…..
Studies in pregnant mice indicate that progesterone (but not 17-OHPC) could prevent preterm delivery…
One study examined the effect of 17-OHPC on cervical length in patients with a history of ≥ 1 preterm births who were allocated to receive 17-OHPC vs an untreated control group.67 No difference in cervical length measurements over time was observed in women who received 17-OHPC. In contrast, vaginal progesterone reduced the rate of cervical shortening in patients with a history of preterm birth or premature cervical shortening…"
Romero R, Stanczyk FZ. Progesterone is not the same as 17α-hydroxyprogesterone caproate: implications for obstetrical practice. Am J Obstet Gynecol. 2013 Jun;208(6):421-6. doi: 10.1016/j.ajog.2013.04.027. Epub 2013 Apr 30. PMID: 23643669; PMCID: PMC4120746.
Levonorgestrel
Levonorgestrel is derived from testosterone, so has androgenic properties, this can affect a female foetus adversely. It can increase the risk of an ectopic pregnancy too.
Levonorgestrel also causes a 50% reduction in SHBG (sex hormone binding globulin). With less SHBG available, both testosterone and oestrogen increase.
The reduction in SHBG allows free testosterone to rise. Testosterone causes scalp hair loss, facial hair, oily skin and acne.
"Levonorgestrel, also known as the morning-after pill, is a first-line oral emergency contraceptive pill with approval from the World Health Organization to prevent pregnancy. It is FDA-approved to be used within 72 hours of unprotected sexual intercourse or when a presumed contraceptive failure has occurred. There have been cases of off-label efficacy for up to 96 hours. A prescription is not needed, and it is available over the counter at local pharmacies. The FDA has also approved levonorgestrel availability for all age groups due to its lack of life-threatening contraindications and side-effect profile. Levonorgestrel can be used as an oral combination pill with estradiol as a long-term option for birth control and is available in other forms, such as implants or transdermal patches. There is a levonorgestrel-releasing intrauterine device considered to be a “low maintenance” birth control option for women that is efficacious for up to five years. It has also been used off-label effects to treat endometrial hyperplasia, menorrhagia, endometriosis, and menopausal hormone therapy."
-Vrettakos C, Bajaj T. Levonorgestrel. [Updated 2023 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539737/-
"To determine the effect of long-acting intrauterine progestin on the impedance to blood flow in the uterine arteries, we applied a levonorgestrel-releasing (20 microg/day) intrauterine contraceptive system (LNG-IUS) to the uterine cavity of 27 fertile, regularly menstruating women. Measurements were performed using transvaginal colour Doppler ultrasonography in the mid-luteal phase and on day 1 of menstruation in the absence of the LNG-IUS and 3 months later in its presence. The mean (SD) mid-luteal uterine artery pulsatility index (PI) had risen from a pretreatment level of 2.28 (0.48) to 2.70 (0.67) after 3 months use of the LNG-IUS (P < 0.01), but as regards day 1 of menstruation, no change was observed. The increase in the mean PI (SD) at the mid-luteal phase was present only in subjects with serum levonorgestrel concentrations >200 pg/ml (n = 16) [pretreatment, 2.25 (0.47); with LNG-IUS, 2.83 (0.59), P < 0.001], and absent in those with serum levonorgestrel concentrations <200 pg/ml (n = 10) [pretreatment, 2.31 (0.53); with LNG-IUS, 2.51 (0.79), ns]. Mean progesterone concentrations were lower with the LNG-IUS in place in both groups. We conclude that a levonorgestrel-releasing intrauterine device appears to increase the impedance to blood flow in the uterine arteries during the mid-luteal phase in correlation with serum concentrations of LNG and a concomitant decrease in serum progesterone concentrations."
-I Järvelä, A Tekay, P Jouppila, The effect of a levonorgestrel-releasing intrauterine system on uterine artery blood flow, hormone concentrations and ovarian cyst formation in fertile women., Human Reproduction, Volume 13, Issue 12, 1 December 1998, Pages 3379–3383, https://doi.org/10.1093/humrep/13.12.3379-
Occurrence of Levonorgestrel in Water Systems and Its Effects on Aquatic Organisms: A Review
"Levonorgestrel is one of the active ingredients of oral contraceptives detected in the aquatic environment at concentrations in the order of ng/L. During the past decade, a wealth of new information about levonorgestrel has been produced, with several studies having reported negative effects in the reproduction and growth of aquatic organisms after exposure to this emerging contaminant of concern." In the present study, the data about its levels in water and its effects on aquatic organisms were integrated and used to perform an updated preliminary aquatic risk assessment for levonorgestrel based on the guideline for Environmental Risk Assessment of Medicinal Products for Human Use from the European Medicines Agency. The aim was to investigate if this pharmaceutical has a risk for adverse effects on aquatic organisms (i.e. for organisms residing in surface water and groundwater). The results evidenced that levonorgestrel is likely to pose an environmental risk to surface water (risk quotient >1). Based on these results, a more refined risk assessment for this pharmaceutical is needed. Besides, our findings highlight the need for investigation under the adverse outcome pathway (AOP) framework, as well as for further studies about toxicological interactions between levonorgestrel and other synthetic steroids."
-Oropesa AL, Guimarães L. Occurrence of Levonorgestrel in Water Systems and Its Effects on Aquatic Organisms: A Review. Rev Environ Contam Toxicol. 2021;254:57-84. doi: 10.1007/398_2020_44. Erratum in: Rev Environ Contam Toxicol. 2021;254:217. doi: 10.1007/398_2020_52. PMID: 32494900.-
Ovarian function after seven years' use of a levonorgestrel IUD
"Fifteen women with regular menstrual periods and seven amenorrheic women who had been using a levonorgestrel-releasing (LNg) IUD for more than seven years were studied. For controls, eight women using TCu380Ag IUDs, for more than seven years were studied during two complete menstrual cycles. Ovarian function was assessed with hormonal determination and ultrasound examinations. The regularly menstruating women were studied for two complete menstrual cycles and the amenorrheic women for eight weeks.
In the regularly menstruating LNg-IUD users, according to progesterone levels, 93% of the cycles were ovulatory but just 58% of these ‘ovulatory’ cycles showed normal follicular growth and rupture. Follicular cysts and luteinization of regressing follicles were observed in 42% of the 26 ‘ovulatory’ cycles studied."
"It is concluded that LNg-IUDs may exert a contraceptive effect in many different ways, such as inhibition of ovulation, endometrial changes preventing implantation, alteration of physical and chemical properties of cervical mucus affecting sperm transport and subtle disturbances in hypothalamic pituitary ovarian function, resulting in alterations of follicular development and rupture."
-Barbosa, I., Olsson, S.E., Odlind, V. et al. Ovarian function after seven years' use of a levonorgestrel IUD.
Adv Contracept 11, 85–95 (1995). https://doi.org/10.1007/BF01987274-
“Currently available oral contraceptives increased the risk of venous thrombosis fivefold compared with non-use (odds ratio 5.0, 95% CI 4.2 to 5.8). The risk clearly differed by type of progestogen and dose of oestrogen. The use of oral contraceptives containing levonorgestrel was associated with an almost fourfold increased risk of venous thrombosis (odds ratio 3.6, 2.9 to 4.6) relative to non-users, whereas the risk of venous thrombosis compared with non-use was increased 5.6-fold for gestodene (5.6, 3.7 to 8.4), 7.3-fold for desogestrel (7.3, 5.3 to 10.0), 6.8-fold for cyproterone acetate (6.8, 4.7 to 10.0), and 6.3-fold for drospirenone (6.3, 2.9 to 13.7). The risk of venous thrombosis was positively associated with oestrogen dose. We confirmed a high risk of venous thrombosis during the first months of oral contraceptive use irrespective of the type of oral contraceptives.
Conclusions: Currently available oral contraceptives still have a major impact on thrombosis occurrence and many women do not use the safest brands with regard to risk of venous thrombosis.”
-van Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. BMJ. 2009 Aug 13;339:b2921. doi: 10.1136/bmj.b2921. PMID: 19679614; PMCID: PMC2726929.-
"The continuous use of synthetic hormones as contraceptive pill or hormonal replacement therapy among women is increasing day by day. The widespread use of different formulations as oral contraceptives by women throughout their reproductive cycle has given rise to a serious concern for studying the effects of oral contraceptives on enzymatic profile and DNA damage in peripheral blood lymphocytes among users."
"The results of the present study suggest that OCs not only effects enzymatic activity but also results in DNA damage that may vary with the duration of using oral contraceptives. A significant increase in LDH, GGT, SGPT, SGOT, globulin and decrease in ALP as well as albumin was found among users as compared to non-users. The observed DNA damage was more in users as compared to non-users. Hormonal contraceptives seem to exert DNA damage and also have significant effects on blood serum enzymes."
-Naz F, Jyoti S, Rahul, Akhtar N, Siddique YH. Effect of Oral Contraceptive Pills on the Blood Serum Enzymes and DNA Damage in Lymphocytes Among Users. Indian J Clin Biochem. 2016 Jul;31(3):294-301. doi: 10.1007/s12291-015-0533-x. Epub 2015 Dec 8. PMID: 27382200; PMCID: PMC4910851.-
Medroxyprogesterone / Medroxyprogesterone Acetate
Medroxyprogesterone acetate is the most commonly used synthetic progestin.
Medroxyprogesterone acetate exacerbates glutamate excitotoxicity
"We previously demonstrated that progesterone functions as a neuroprotective agent whereas medroxyprogesterone acetate (MPA; Provera) does not. Moreover, MPA antagonized the neuroprotective and neurotrophic outcomes induced by 17beta-estradiol (E2)."
"Results of these analyses indicated that both crystalline MPA and a pharmaceutical formulation (Depo-Provera) lacked neuroprotective efficacy, indicating that the effects were not dependent upon MPA formulation. Likewise, MPA in the prevention and treatment paradigms were equally ineffective at promoting neuronal survival, indicating that timing of MPA administration was not a factor. Further, the detrimental effects of MPA were not due to the presence of the acetate group, as medroxyprogesterone was as ineffective as MPA in promoting neuronal survival. Moreover, MPA pretreatment exacerbated neuron death induced by glutamate excitotoxicity as indicated by a 40% increase in neuron death determined by direct live/dead cell count and a commensurate increase in the number of positive cells by terminal deoxynucleotidyl transferase-mediated nick end-labeling. Collectively these results predict that the progestin formulation of hormone therapy will affect the vulnerability of the central nervous system to degenerative insults."
-Nilsen J, Morales A, Brinton RD. Medroxyprogesterone acetate exacerbates glutamate excitotoxicity. Gynecol Endocrinol. 2006 Jul;22(7):355-61. doi: 10.1080/09513590600863337. PMID: 16864144.-
"Background: Use of menopausal hormonal therapy (MHT)-containing estrogen and a synthetic progestin is associated with an increased risk of breast cancer. It is unclear if progesterone in combination with estrogen carries a lower risk of breast cancer. Limited data suggest differences between progesterone and progestins on cardiovascular risk factors, including cholesterol and glucose metabolism. Whether this translates to differences in cardiovascular outcomes is uncertain. We conducted a systematic review and meta-analysis to synthesize the existing evidence about the effect of progesterone in comparison to synthetic progestins, each in combination with estrogens, on the risk of breast cancer and cardiovascular events.
Methods: We searched MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and Scopus through 17 May 2016 for studies that enrolled postmenopausal women using progesterone vs. synthetic progestins and reported the outcomes of interest. Study selection and data extraction were performed by two independent reviewers. Meta-analysis was conducted using the random effects model.
Results: We included two cohort studies and one population-based case-control study out of 3410 citations identified by the search. The included studies enrolled 86,881 postmenopausal women with mean age of 59 years and follow-up range from 3 to 20 years. The overall risk of bias of the included cohort studies in the meta-analysis was moderate. There was no data on cardiovascular events. Progesterone was associated with lower breast cancer risk compared to synthetic progestins when each is given in combination with estrogen, relative risk 0.67; 95 % confidence interval 0.55-0.81.
Conclusions: Observational studies suggest that in menopausal women, estrogen and progesterone use may be associated with lower breast cancer risk compared to synthetic progestin."
-Asi N, Mohammed K, Haydour Q, Gionfriddo MR, Vargas OL, Prokop LJ, Faubion SS, Murad MH. Progesterone vs. synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016 Jul 26;5(1):121. doi: 10.1186/s13643-016-0294-5. PMID: 27456847; PMCID: PMC4960754.-
In Defense of Progesterone: A Review of the Literature
"Context • The medical literature on the use of progesterone in postmenopausal women is often confusing and contradictory. Some physicians implicate natural progesterone in an increase in the risk of breast cancer. The chemical structure of natural progesterone (P4) is quite different from chemically altered, synthetic chemicals called progestins, which results in different actions at the cell level. Objective • The research team intended to review the literature to examine the benefits and safety of natural progesterone and determine whether it can cause an increase or decrease in breast cancer risk. Design • A review of the medical literature to examine the benefits and safety of natural progesterone as compared with synthetic progestins. Intervention • Studies examined compared controls not receiving hormone therapy with women receiving estrogen alone and in combination with natural progesterone and with various synthetic progestins, such as medroxyprogesterone acetate-the most commonly used synthetic progestin. Outcome Measures • Outcome measures included factors such as progression and survival of breast and other cancers and other epidemiological and laboratory data. Results • A meta-analysis of 3 studies involving 86 881 postmenopausal women reported that the use of natural progesterone was associated with a significantly lower risk of breast cancer compared with synthetic progestins. Anovulation and low levels of serum progesterone have been associated with a significantly higher risk of breast cancer in premenopausal women. Use of progesterone has been linked to lower rates of uterine and colon cancers and may also be useful in treating other cancers such as ovarian, melanoma, mesothelioma, and prostate. Progesterone may also be helpful in preventing cardiovascular disease and preventing and treating neurodegenerative conditions such a stroke and traumatic brain injury. Conclusions • Physicians should have no hesitation prescribing natural progesterone. The evidence is clear that progesterone does not cause breast cancer. Indeed, progesterone is protective and preventative of breast cancer."
-Lieberman A, Curtis L. In Defense of Progesterone: A Review of the Literature. Altern Ther Health Med. 2017 Nov;23(6):24-32. PMID: 29055286.-
Medroxyprogesterone interferes with ovarian steroid protection against coronary vasospasm
"Cardiovascular disease, the major cause of death in post-menopausal women, can be reduced by replacement of ovarian steroid hormones. To compare medroxyprogesterone with progesterone as the progestin in hormone replacement therapy from the standpoint of coronary artery vasospasm, we treated ovariectomized rhesus monkeys with physiological levels of estradiol-17 beta in combination with medroxyprogesterone or progesterone for four weeks. Coronary vasospasm in response to pathophysiological stimulation without injury showed that progesterone plus estradiol protected but medroxyprogesterone plus estradiol failed to protect, allowing vasospasm. We conclude that medroxyprogesterone in contrast to progesterone increases the risk of coronary vasospasm."
-Miyagawa K, Rösch J, Stanczyk F, Hermsmeyer K. Medroxyprogesterone interferes with ovarian steroid protection against coronary vasospasm. Nat Med. 1997 Mar;3(3):324-7. doi: 10.1038/nm0397-324. PMID: 9055861.-
The Women's Health Initiative Memory Study: findings and implications for treatment
“There were two study arms, one involving 4532 postmenopausal women who received continuous combined oestrogen (conjugated equine oestrogens [CEE] plus medroxyprogesterone acetate) or placebo, and the other involving 2947 hysterectomised women randomised to continuous unopposed CEE or placebo. All participants were age 65 years or older. CEE with or without medroxyprogesterone acetate, given to women age 65 years and older, does not protect against dementia or cognitive decline, but substantially increases the risk of dementia of any cause and cognitive decline.”
-Dr Michael C Craig, MRCPsych m.craig@iop.kcl.ac.uk ∙ Pauline M Maki, PhDc ∙ Prof Declan GM Murphy, MD, The Lancet Neurology, Volume 4, Issue 3, 190 - 194, DOI: 10.1016/S1474-4422(05)01016-1-
"In order to assess the androgenic activity of synthetic "progestins" currently used as "antiandrogens" for the treatment of prostate cancer in men..."
"..Medroxyprogesterone acetate (MPA) is almost equipotent with 5 alpha-dihydrotestosterone (DHT), a 49% increase in prostatic weight being observed at the low dose of 0.15 mg, twice daily (P less than 0.01). Megestrol acetate (Megace), chlormadinone acetate (CMA) and spironolactone were less potent but caused a 36-59% increase in prostatic weight at the highest dose used, namely 10 mg. At the 5 mg dose, cyproterone acetate (CPA) caused a 75% increase in prostatic weight. The androgenic activity of the compounds is even more clearly illustrated by their marked stimulatory effect on prostatic ornithine decarboxylase (ODC) activity. MPA, at the low dose of 0.15 mg, caused a 20-fold increase (relative to the effect of placebo) in the activity of the enzyme while the same dose of DHT caused a 15-fold stimulation of enzymatic activity. At the 10 mg dose, megestrol acetate, CMA and spironolactone caused 13.1, 11.8 and 8.6-fold stimulations of ODC activity, respectively. Flutamide, on the other hand, had no stimulatory effect on either ventral prostate weight or prostatic ODC activity. In agreement with glucocorticoid activity, MPA, megestrol acetate and CMA caused a marked inhibition (45-64%) of adrenal weight. The present data show that MPA is a highly potent androgen while megestrol acetate, CMA, CPA and spironolactone have lower but significant androgenic activity on all the parameters measured. It should be added that MPA, megestrol acetate and CMA are completely devoid of antiandrogenic activity while spironolactone shows weak antiandrogen action and CPA is a mixed agonist-antagonist. Flutamide, the compound used as reference, is the only compound devoid of any androgenic action and is thus acting as a pure antiandrogen on both ventral prostate weight and prostatic ODC activity. The present data have major implications for the choice of drug to be used for the treatment of androgen-sensitive diseases, especially prostate cancer. As shown by the present data, the synthetic "progestins" so-far available all possess variable levels of androgenic activity and are thus not recommended for the treatment of prostate cancer."
-Labrie C, Cusan L, Plante M, Lapointe S, Labrie F. Analysis of the androgenic activity of synthetic "progestins" currently used for the treatment of prostate cancer. J Steroid Biochem. 1987 Oct;28(4):379-84. doi: 10.1016/0022-4731(87)91054-5. PMID: 2444770.-
"It has been shown that ovarian steroid hormones can reduce the incidence of cardiovascular disease in postmenopausal women. As hormone replacement therapy for postmenopausal women, progestins are added to estrogens to eliminate the increased risk of endometrial cancer. However, the effects of progestins on the atherogenic process have not been well understood. In the present study, we examined the effects of progestins on the expression of vascular cell adhesion molecule-1 (VCAM-1) in human umbilical vein endothelial cells (HUVECs). Immunocytochemical analysis revealed the presence of progesterone receptors in HUVECs. Progesterone clearly inhibited tumor necrosis factor-α–activated expression of VCAM-1 protein and its mRNA in HUVECs. Synthetic progesterone receptor agonist R5020 also inhibited the tumor necrosis factor-α–activated VCAM-1 expression, whereas medroxyprogesterone acetate (MPA) failed to do so. Electrophoretic mobility shift assays demonstrated that progesterone, but not MPA, inhibited DNA binding of the transcription nuclear factor-κB, which is critical for the inducible expression of VCAM-1. Because the expression of VCAM-1 is one of the earliest events that occurs in the atherogenic process, this adhesion molecule might be a target molecule for progesterone on vascular walls. The contrasting effects of progesterone and MPA seem clinically important, inasmuch as MPA is a widely used progestin in the regimen of hormone replacement therapy."
-Michio Otsuki, Hiroshi Saito, Xin Xu, Satoru Sumitani, Haruhiko Kouhara, Tadamitsu Kishimoto, and Soji Kasayama; Arteriosclerosis, Thrombosis, and Vascular Biology, Volume 21, Number 2, https://doi.org/10.1161/01.ATV.21.2.243.-
 Feeling tired, foggy, or struggling with stubborn weight gain—especially around the waist? You might be surprised to learn that these symptoms could be linked to insulin resistance, a condition that a…
Feeling tired, foggy, or struggling with stubborn weight gain—especially around the waist? You might be surprised to learn that these symptoms could be linked to insulin resistance, a condition that a… Are you struggling with irregular cycles, unwanted hair growth, or unexplained fatigue? You’re not alone. Polycystic Ovarian Syndrome (PCOS) affects up to 10% of women of reproductive age—and many mor…
Are you struggling with irregular cycles, unwanted hair growth, or unexplained fatigue? You’re not alone. Polycystic Ovarian Syndrome (PCOS) affects up to 10% of women of reproductive age—and many mor… While progesterone is often discussed in relation to reproductive health, emerging research reveals its remarkable role in supporting brain function and protecting against neurological decline. Proges…
While progesterone is often discussed in relation to reproductive health, emerging research reveals its remarkable role in supporting brain function and protecting against neurological decline. Proges… Incase you missed it!
Today is the last day for you to claim 15% off our Natpro 100ml Dispensers. The sale ends at midnight tonight.
How to Claim Your 15% Discount:
•Shop at
Incase you missed it!
Today is the last day for you to claim 15% off our Natpro 100ml Dispensers. The sale ends at midnight tonight.
How to Claim Your 15% Discount:
•Shop at